|
Empowering healthcare
through technology transfer
IMT
Innovations, a Swiss
med-tech company with over
30 years of experience in
mechanical ventilation, is
redefining how critical-care
ventilators are brought to
market. Rather than
exporting finished devices,
IMT offers a unique
technology licensing model-
providing U.S. partners with
full access to the IP,
engineering, and
manufacturing know-how to
locally produce
next-generation ventilators.
At the heart of this
program is the “box”
ventilator - a newly
developed, AI-ready
life-support ventilator
designed for ICU and
long-term care settings.
Engineered to meet FDA
510(k) and EU MDR standards.
Thanks to the project's
advanced stage, the FDA
approval process can begin
by mid-2026 at the latest.
The box is built for high
performance, supply chain
independence, and long-term
resilience in national
healthcare systems.
Harri Friberg, CEO of
IMT Innovations, shares
insights into the concept
and how U.S. manufacturers
can take part.
Mr. Friberg, what
are the outstanding
technical features of the
“box” ventilator that make
it relevant in clinical
practice?
Answer: Our
focus was on a combination
of high performance, ease of
use, and reliability. The
device offers a peak flow of
over 280 L/min and is
suitable for invasive and
non-invasive ventilation of
adults and children. A key
feature is the adaptive
trigger technology, combined
with advanced algorithms,
which ensure optimal
patient-ventilator
synchrony. This reduces the
work of breathing for the
patient and improves
comfort, which is crucial in
non-invasive applications
and during weaning. The
modular design also allows
for future hardware and
software expansions. For
clinical staff, we have
developed an intuitive,
touch-optimised user
interface that significantly
simplifies setup and
monitoring.
Instead of distributing the
ventilator yourself, you're
offering a licensing model.
What does that include, and
who is it designed for?
Answer:
Our mission is to make
advanced ventilation
technology locally
accessible - anywhere in the
world. Through our licensing
model, we provide partners
with a complete technology
transfer package. This
includes the full technical
documentation, manufacturing
protocols, regulatory
pathways, and supplier
network needed to produce
and scale the ventilator
independently.
Licensees receive the rights
to manufacture, further
develop, register, and sell
the device within their
territory - under their own
name. We support them
through every step, from
regulatory approvals
(including FDA 510(k) or CE
marking) to production setup
and technical training.
This business model is
designed for
forward-thinking med-tech
companies, healthcare
manufacturers, or
government-backed
initiatives looking to
secure a strategic,
self-sufficient supply of
critical care technology.
Ultimately, the licensee
becomes the legal
manufacturer for their
region - owning both the
production and the future of
the product.
What strategic advantages
does this model offer
licensees, especially
regarding global supply
chains and import tariffs?
Answer:
The COVID-19 pandemic has
exposed the vulnerability of
traditional supply chains.
Our model creates
independence from foreign
manufacturers and their
delivery capacities. Local
production not only
eliminates long transport
routes but also potentially
high import tariffs, thereby
significantly reducing
costs. The decisive point,
however, is the complete
transfer of intellectual
property (IP). The licensee
not only acquires a
production license but also
gains control over the
product design and can even
develop the device
independently. They thus own
their own ventilator. This
strengthens the local
economy, creates highly
qualified jobs, and ensures
sustainable, long-term
healthcare for its own
population.
|
|
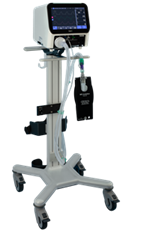
A box License provides partners with a
complete technology
transfer package.
This includes the
full technical
documentation,
manufacturing
protocols,
regulatory pathways,
and supplier network
needed to produce
and scale the
ventilator
independently.
|
The box Ventilator at a
Glance
|
Feature
|
Specification
|
|
Application
|
Adults & Children
(invasive/non-invasive),
O2 Therapy
|
|
Performance
|
> 280 L/min Peak
Flow
|
|
Synchronization
|
Adaptive Trigger
Technology for
optimal
patient-ventilator
synchrony
|
|
Operation
|
Intuitive,
touch-optimised user
interface
|
|
Design
|
Modular, future-proof
system (hardware &
software)
|
|
Regulatory
|
Compliant with FDA
510(k) & MDR/CE
|
|
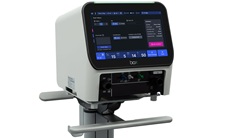
|
The box ventilator
is licensed to
interested regional
manufacturers. It is
designed for
invasive and
non-invasive
ventilation of
children and adults.
|
The New Distribution
Concept (Licensing) at a
Glance
|
Factor
|
Description
|
|
Rights
|
Manufacturing &
distribution rights
for the defined
region
|
|
Support
|
Comprehensive support
for regulatory
training, and supply
chain
|
|
Strategic Advantage
|
Independence from global
supply chains,
avoidance of tariffs
|
|
IP-Transfer
|
Complete transfer of
intellectual
property; the
licensee becomes the
“Legal Manufacturer”
with their own
product design
|
 PRINT
THIS ARTICLE
PRINT
THIS ARTICLE
|





